
Antidiabetic activity of N-(6-substituted-1,3. Moreno-Díaz, H., Villalobos-Molina, R., Ortiz-Andrade, R., Díaz-Coutiño, D., Medina-Franco, J. Investigación y desarrollo de nuevos medicamentos: de la molécula al farmaco. Journal American Industrial Hygiene Association Quarterly, 10, 94-97. Ciudad de la Habana: Editorial Universitaria. Barcelona, España: Reverté S.A.Įscalona, J., Carrasco, R., & Padrón, J. Retrieved 11 de 10 de 2016 from ĭurst, H. American Scientist, 91, 138-149.īerman, H., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T., Weissig, H., et al. In many ways, the fight against antibiotic resistance is already lost preventing bacterial disease requires thoughtful new approaches. Revista Medica Herediana, 24, 156-161.Īmábile-Cuevas, C. Infecciones en huéspedes inmunocomprometidos. Advanced Drug Delivery Reviews, 46, 3-26.Ĭuellar, L. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Journal of Chemical Information and Modeling, 52, 3099-3105.
#PBP3 PDB FREE#
admetSAR: A Comprehensive Source and Free Tool for Assessment. The most promising compounds will be evaluated by in vitro assays as potential antibacterial agents.Ĭheng, F., Li, W., Zhou, Y., Shen, J., Wu, Z., Liu, G., et al. All the obtained compounds were fully characterized by IR spectroscopy, as well as mono- and bidimensional NMR techniques.
Finally, assays were carried out aiming to the formation of β-lactam rings, using the Staudinger-type cycloaddition reaction of 2-chloroacetyl chloride with cyclic imines. The thiazolidine-4-ones were obtained from the aminopyrimidines synthesized above, using three-component cyclocondensation reactions with 2-mercaptoacetic acid and benzaldehyde, in anhydrous toluene or benzene as solvents and using conditions of reflux with Dean- Stark.
#PBP3 PDB SERIES#
The pyrimidine series were synthesized starting by the reaction of chalcones and guanidine, giving rise to the corresponding amonopyrimidines, which were then reacted with aromatic and heteroaromatic aldehydes to obtain the acyclic azomethine compounds.
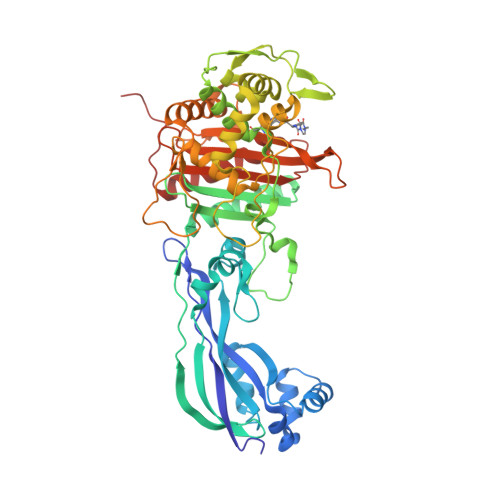
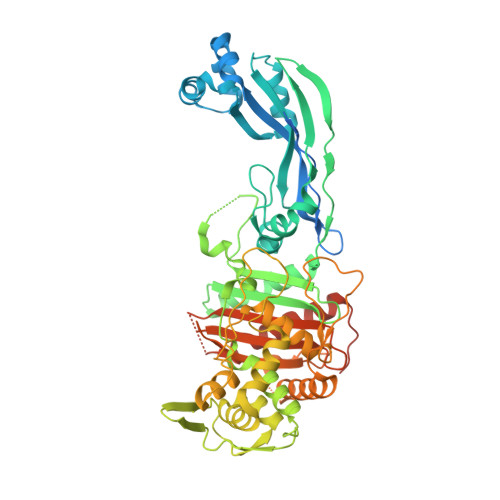
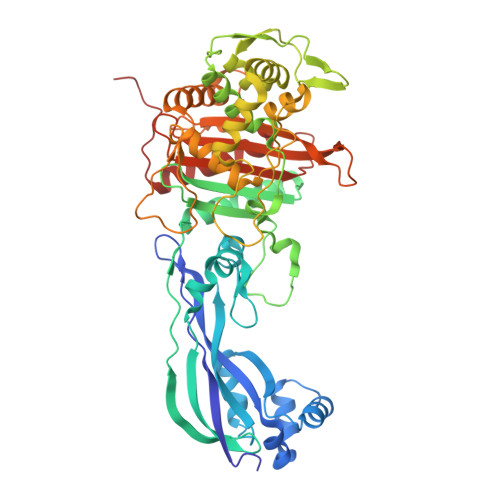
The dihydropyrazole derivatives were obtained from the reaction of chalcones with one equivalent of hydrazine derivatives by one-step cyclocondensations. In general, the synthesis of the selected compounds was carried out from the α,β-unsaturated carbonyl compounds as precursors. By these studies, 8 compounds were selected by their binding energies (from -5,36KJ/mol to – 7,05KJ/mol) and significant interactions with the amino acids of the receptor in its active site. Key physicochemical properties were calculated (absorption, distribution, metabolism, excretion and toxicity), to determine the bioavailability of the designed compounds and to perform a preselection of 12 derivatives which were then optimized and studied by molecular docking with the receptor PBP3 (4bjp) from Escherichia coli. In this paper, a set of computational tools were used to design and evaluate molecular structures resulting from the combination of the biologically interesting pyrazoline, aminopyrimidine and thiazolidine nuclei (molecular modification) to obtain new bioactive compounds.


 0 kommentar(er)
0 kommentar(er)
